We’re always looking for new ways to charge our mobile gadgets. Wind, sunlight, fuel cells, you name it. These all seem to rely on an external device pushing power to a rechargeable battery. However, what if the charging mechanism was built into the battery itself? That’s the idea behind this wind-up Charge Batteries.
Yes, I did just say wind-up batteries, and it’s exactly what you’re thinking. Imagine a AA battery that you can wind in order to generate a charge. Do your fingers hurt yet? I’ve had hand-cranked flashlights before, and I remember how long it took to get even a few minutes worth of light. Can you imagine twisting a pair of tiny batteries for extended periods? Something tells me that this concept will never be turned into reality.


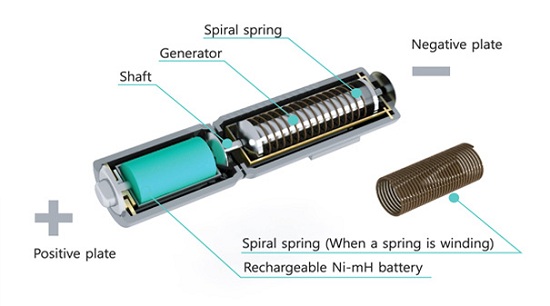



The question here isn't whether or not this would 'work': clearly, the physics are sound. However, will it work efficiently enough to be of any use to anyone? I'm a little rusty, but let's try and run some numbers (feel free to jump in and correct me if I go wrong).
Estimating some spring dimensions (0.7mm wire diameter, 13mm outer diameter, 30mm free length) and looking up comparable springs from an online supplier (http://www.leespring.com/browse_catalog.asp?rbunitOfMeasure=…) seems to indicate a spring constant (k) of around 0.2 N/mm (200 N/m).
Hooke's law states that the potential energy stored in a spring is equal to (k(x^2))/2, where k is the spring constant and x is the displacement (distance squeezed/stretched). If we take a wild, optimistic guess that each 360 degree rotation of the battery head produces 1cm of compression in the spring, that means each rotation stores a nicely round (200(.01^2))/2 = 0.01J of potential energy in the spring.
Let's continue our optimism and say that the mechanism connected to the spring is able to quickly convert that compression into rotation, spinning a tiny generator which converts the kinetic energy into electric potential energy in the battery at, let's say, 80% efficiency. Each twist therefore stores 0.008J, or 0.002 mWh, in the battery.
A decent NiMH AA battery stores around 2500 mAh. If we assume the cell in the design maintains the same energy density as a normal NiMH battery, at ~30% of the size, we can estimate a fully charged capacity of ~750 mAh. Since AA batteries operate at 1.5V, this gives us a total energy capacity of (1.5 x 750) = 1125 mWh.
This means that, to fully charge the battery, you'd need to twist the top all the way around (1125/.002) = 562,500 times. Based on my home experiments fondling the top of a normal AA battery, I estimate that it's possible to rotate the top approximately twice per second, which means that fully charging the battery would take (((562 500 / 2) / 60) / 60) / 24 = 3.25 days of continuous twisting. (note: this ignores wrist and elbow fatigue, which I started to experience after only a few hundred twists)
So more than 3 straight days of twisting one of these suckers, for a third of the power of a standard AA battery. I'd call that unfeasible, unless you had some serious time on your hands and were absolutely desperate for a few milliwatts.